Provide Patients Another Option for Relief
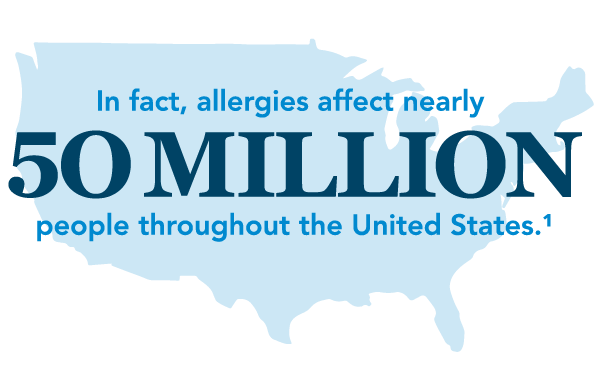
Allergies are a burdensome condition for many people in the US.
What is Allergy Immunotherapy?
Allergy immunotherapy (AIT), along with allergen avoidance and symptomatic pharmacotherapy, is considered a useful treatment modality in the management of allergic conditions.2
By providing AIT at your practice, you can offer patients another proven treatment option that may lead to relief of allergy symptoms.3
How does AIT Work?
AIT is a long-term treatment for allergies.2
Researchers believe that over time, allergy immunotherapy helps the patient’s immune system develop resistance to allergens and reduces the ability to cause allergic symptoms.2,4,5
Patients are administered gradually increasing doses of symptom-causing allergen(s) to train the immune system to reduce the allergic response when exposed to the trigger allergen(s).6
It is believed that the incremental increases of the allergen(s) given to the patient causes7
Although the precise mechanism of action of AIT is unknown, research suggests there is the potential for long-term sustained relief of allergy symptoms. Randomized, double-blind, placebo-controlled trials demonstrate efficacy of AIT for up to 3 years.3,8-13
For many patients, subcutaneous AIT may be an effective option with an established safety profile
Subcutaneous AIT is appropriate when an allergy is established and the patient cannot avoid exposure to the allergen. Stallergenes Greer allergenic extracts are approved for use in subcutaneous allergy immunotherapy for reduction of allergen-induced allergic symptoms. Standardized Cat and Standardized Mite allergenic extracts are approved for treatment of rhinitis, conjunctivitis, and allergic asthma. Please see product-specific package inserts for complete indications.12,14,15
Treatment with allergenic extracts has been shown to reduce allergic symptoms of a perennial or seasonal nature such as12,14-18


Additionally, across multiple randomized trials and meta-analyses, subcutaneous AIT has been shown to 15,16,19-23
- Help control symptoms
- Help reduce the need for other medications
Subcutaneous AIT has an established safety profile in both children and adults.19,24,25
The administration of subcutaneous AIT has been associated with severe, life-threatening systemic reactions, including anaphylaxis and death.12,26,27
Some common systemic reactions of subcutaneous AIT include pruritus, nausea, vomiting, and urticaria.12,14-18,26,27
Other reactions include12,14-18,26,27
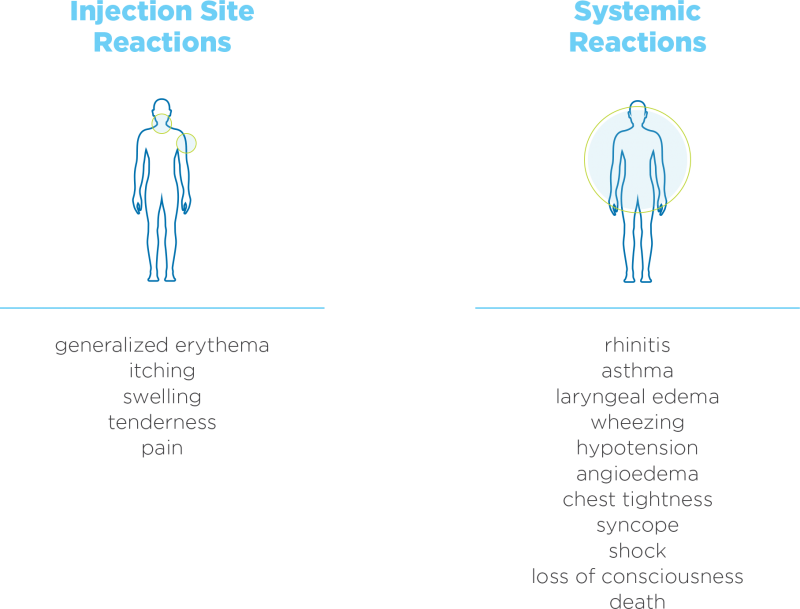
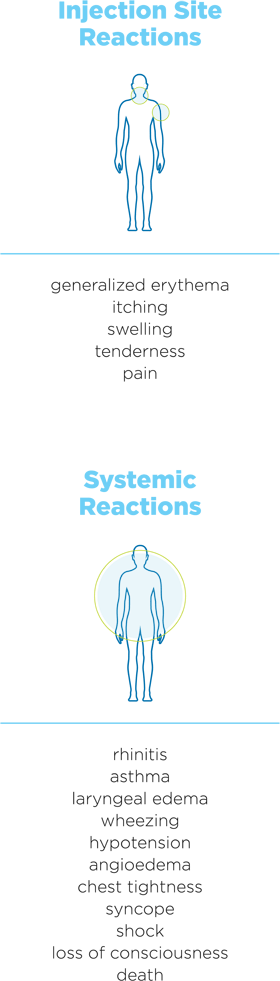
Systemic adverse reactions as a result of subcutaneous AIT have been observed in less than 7% of treated patients, with a much lower percentage (0.2% total) experiencing symptoms classified as severe and very severe.28-30
Always refer to the package insert of the particular subcutaneous AIT product for applicable contraindications, warnings and precautions, drug interactions, and other Important Safety Information.
Is AIT Right for Your Patients?
Candidates who may be appropriate for AIT are patients
- Who require high medication doses, multiple medications, or both to maintain control of their allergy symptoms19
- Who may be dissatisfied with symptomatic pharmacotherapy19
- Who want a treatment that may reduce the long-term use of symptomatic pharmacotherapy19
- Who are unable to avoid confirmed allergens19
Patients who are not appropriate candidates are those
- With severe, unstable, or uncontrolled asthma19
- Who are receiving treatment for cardiovascular disease19
- Who may be at high risk for severe systemic or local allergic reactions19
What AIT Treatment Options are Available?
Subcutaneous AIT, also known as an allergy shot, is the most common form of AIT.31
Allergy shots, or injections, are given in increasing doses of a single or allergen mixture, customized for the patient, composed of the relevant allergen(s) to which the patient is sensitized.32


Stallergenes Greer also offers a sublingual grass allergy immunotherapy tablet treatment option. Learn more.
Whatever treatment option you and your patients decide is appropriate, remember to give clear guidance about 34
- Avoiding treatment interruptions
- Managing adverse reactions
- When to consult a physician
Talking To Your Patients About AIT

Before prescribing AIT, talk to your patients about
- Potential benefits, risks, and costs
- Expected onset of efficacy
- Duration of treatment
- The importance of adhering to the immunotherapy schedule19
Helpful tips for discussing AIT
During the conversation, remember to consider your patient’s
- Perceived effectiveness of treatment, or perceived lack of need to continue treatment
- Experience with any adverse events due to AIT
- Concurrent health problems
- Health insurance coverage
- Travel time to appointments

IMPORTANT SAFETY INFORMATION
WARNING: SEVERE ALLERGIC REACTIONS
- Allergenic extracts can cause severe life-threatening systemic reactions, including anaphylaxis.
- Do not administer allergenic extracts to patients with severe, unstable, or uncontrollable asthma.
- Observe patients in the office for at least 30 minutes following treatment. Emergency measures and personnel trained in their use must be available immediately in the event of a life-threatening reaction.
- Immunotherapy may not be suitable for patients with certain underlying medical conditions that may reduce their ability to survive a systemic allergic reaction.
- Allergenic extracts may not be suitable for patients receiving medications such as beta-blockers that may make them unresponsive to epinephrine or inhaled bronchodilators.
Do not inject intravenously. Initial dose should be based on skin test reactivity.
The most common adverse reactions include localized erythema, itching, swelling, tenderness, and pain. Systemic adverse reactions include generalized skin erythema, urticaria, pruritus, angioedema, rhinitis, wheezing, chest tightness, laryngeal edema, and hypotension.
INDICATIONS
Non-Standardized Allergenic Extracts: Allergenic extracts are indicated for skin test diagnosis of patients with a clinical history of allergies to one or more of the specific allergens and treatment for reduction of allergen-induced allergic symptoms confirmed by positive skin tests or in vitro testing for allergen-specific IgE antibodies. Food extracts have not been proven safe or effective in allergen immunotherapy.
Short Ragweed Pollen Allergenic Extract and Short and Giant Ragweed Pollen Mix Allergenic Extract: Short Ragweed Pollen Allergenic Extract and Short and Giant Ragweed Pollen Mix Allergenic Extract are indicated for skin test diagnosis of patients with a clinical history of allergy to ragweed pollen (Short Ragweed or Short and Giant Ragweed pollen), and immunotherapy for the reduction of ragweed pollen-induced allergic symptoms confirmed by positive skin test or by in vitro testing for pollen-specific IgE antibodies for Short and/or Giant Ragweed pollen.
Standardized Cat Hair Allergenic Extract: Standardized Cat Hair Allergenic Extract is indicated for skin test diagnosis of patients with a history of allergy to cats and treatment of cat hair-induced allergic asthma, rhinitis and conjunctivitis when avoidance is not possible.
Standardized Grass Pollen Allergenic Extracts: Standardized Grass Pollen Allergenic Extracts are indicated for skin test diagnosis of patients with a clinical history of allergy to one or more of the following grass pollens: Bermuda, Kentucky Blue (June), Meadow Fescue, Orchard, Perennial Rye, Redtop, Sweet Vernal, Timothy; and immunotherapy for the reduction of grass pollen-induced allergic symptoms confirmed by positive skin test or by in vitro testing for pollen-specific IgE antibodies for Bermuda, Kentucky Blue (June), Meadow Fescue, Orchard, Perennial Rye, Redtop, Sweet Vernal, or Timothy grass pollens.
Standardized Mite Extracts: Standardized Mite Allergenic Extracts are indicated for diagnosis of skin test reactivity to dust mite allergen and treatment of mite-induced allergic asthma, rhinitis and conjunctivitis in patients that show hypersensitivity to dust mites based on clinical history, allergen exposure history, and skin test reactivity.
Please click here for Package Inserts with full Prescribing Information, including Boxed Warnings.
To report suspected adverse reactions, contact Stallergenes Greer at 1-855-274-1322 or FDA at 1-800-FDA-1088 or www.fda.gov/Safety/MedWatch.
References: 1. Asthma and Allergy Foundation of America. Allergy Facts and Figures. http://www.aafa.org/page/allergy-facts.aspx. Accessed November 1, 2024. 2. Senna G, Ridolo E, Calderon M, Lombardi C, Canonica GW, Passalacqua G. Evidence of adherence to allergen-specific immunotherapy. Curr Opin Allergy Clin Immunol. 2009;9(6):544–548. 3. Bousquet J, Khaltaev N, Cruz AA. ARIA (allergic rhinitis and its impact on asthma) 2008 update in collaboration with the World Health Organization, GA2LEN and AllerGen. Allergy. 2008;63(suppl 86):8-160. 4. Canonica GW. Bousquet J, Casale T, et al. Sub-lingual immunotherapy: World Allergy Organization position paper 2009. Allergy. 2009;64(suppl 91):1-59. 5. American Academy of Allergy, Asthma & Immunology. Statement of the American Academy of Allergy Asthma and Immunology to the House Committee on Energy and Commerce on the 21st Century Cures Initiative. http://www.aaaai.org/Aaaai/media/MediaLibrary/PDF%20Documents/Advocacy/allergy-energy-and-commerce-21st-century-cures-comments.pdf. Accessed November 1, 2024. 6. Incorvaia C, Mauro M, Ridolo E, et al. Patient’s compliance with allergen immunotherapy. Patient Prefer Adherence. 2008;2:247-251. 7. American College of Allergy, Asthma, and Immunology. Allergy immunotherapy. http://acaai.org/allergies/allergy-treatment/allergy-immunotherapy. Accessed November 1, 2024. 8. Durham SR, Walker SM, Varga EM, et al. Long-term clinical efficacy of grass-pollen immunotherapy. N Engl J Med. 1999;341(7):468-475. 9. Jutel M, Agache I, Bonini S, et al. International consensus on (ICON) allergy immunotherapy (AIT). J Allergy Clin Immunol. 2015;136(3):556-568. 10. Li JT, Bernstein DI, Calderon, MA, et al. Sublingual grass and ragweed immunotherapy: clinical considerations—a PRACTALL consensus report. J Allergy Clin Immunol. 2016;137(2):369-376. 11. Giovane AL, Bardare M, Passalacqua G, et al. A three-year double-blind placebo-controlled study with specific oral immunotherapy to Dermatophagoides: evidence of safety and efficacy in paediatric patients. Clin Exp Allergy. 1994;24(1):53-59. 12. ORALAIR® full Prescribing Information, Stallergenes SAS 2018. 13. GRASTEK® full Prescribing Information. Merck & Co, Inc. 2019. 14. Allergenic Extracts Standardized Mite Extract [package insert]. GREER. 2009. 15. Allergenic Extract Standardized Cat Hair [package insert]. GREER. 2015. 16. Allergenic Extract Prescription Set of Serial Dilutions [or Maintenance Vials[s]] [package insert]. GREER. 2004. 17. Allergenic Extracts Short Ragweed and G.S. Ragweed Mix [package insert]. GREER. 2017. 18. Allergic Extracts Pollens, Molds, Epidermals, Insects, Dusts, Foods and Miscellaneous Inhalants [package insert]. GREER. 2023. 19. Cox L, Nelson H, Lockey R, et al. Allergen immunotherapy: a practice parameter third update. J Allergy Clin Immunol. 2011;127(suppl 1):S1-55 20. Abramson MJ, Puy RM, Weiner JM. Allergen immunotherapy for asthma. Cochrane Database Syst Rev. 2003;(4):CD001186. 21. Abramson MJ, Puy RM, Weiner JM. Is allergen immunotherapy effective in asthma?: a meta-analysis of randomized controlled trials. Am J Respir Crit Care Med. 1995;151(4):969-974. 22. Abramson MJ, Puy RM, Weiner JM. Immunotherapy in asthma: an updated systematic review. Allergy. 1999; 54(10):1022-1041. 23. Nanda A, O’Connor M, Anada M, et al. Dose dependence and time course of the immunologic response to administration of standardized cat allergen extract. J Allergy Clin Immunol. 2004;114(6):1339-1344 24. Caminati M, Dama A, Schiappoli M, Senna G. Balancing efficacy against safety in sublingual immunotherapy with inhalant allergens: what is the best approach? Expert Rev Clin Immunol. 2013;9(10):937-47. 25. Drachenberg KJ, Urban E, Pröll S, Woroniecki SR. Sublingual specific immunotherapy for adults and children: a post-marketing surveillance study. Allergol Immunopathol. 2004;32(2):76-81. 26. Allergenic Extracts For Diagnostic Use Only Scratch, Prick, Puncture or Intradermal Testing [package insert]. GREER. 2004. 27. Allergenic Extracts Standardized Grass Pollen Extracts [package insert]. GREER. 2004. 28. Epstein TG, Liss GM, Murphy-Berendts KM, Bernstein DI. Risk factors for fatal and nonfatal reactions to subcutaneous immunotherapy: National surveillance study on allergen immunotherapy (2008-2013). Ann Allergy Asthma Immunol. 2016;116(4):354-359. 29. Greenberg MA, Kaufman CR, Gonzalez GE, Rosenblatt CD, Smith LJ, Summers RJ. Late and immediate systemic-allergic reactions to inhalant allergen immunotherapy. J Allergy Clin Immunol. 1986;77(6):865-870. 30. Ragusa VF, Massolo A. Non-fatal systemic reactions to subcutaneous immunotherapy: a 20-year experience comparison of two 10-year periods. Eur Ann Allergy Clin Immunol. 2004;36(2):52-55. 31. American College of Allergy, Asthma, and Immunology. Allergy Immunotherapy. Allergy Shots. http://acaai.org/allergies/treatment/allergy-shots-immunotherapy. Accessed November 1, 2024. 32. Agency for Healthcare Research and Quality. Subcutaneous and Sublingual Immunotherapy To Treat Allergic Rhinitis/Rhinoconjunctivitis and Asthma. http://www.ncbi.nlm.nih.gov/books/NBK158932/pdf/Bookshelf_NBK158932.pdf. Accessed November 1, 2024. 33. American Academy of Allergy, Asthma & Immunotherapy. Allergy Shots (Immunotherapy). http://www.aaaai.org/conditions-and-treatments/treatments/allergy-shots-(immunotherapy). Accessed November 1, 2024. 34. American College of Allergy, Asthma & Immunology. Sublingual Immunotherapy (SLIT). http://acaai.org/allergies/allergy-treatment/allergy-immunotherapy/sublingual-immunotherapy-slit. Accessed November 1, 2024.
