Order allergen extracts, testing devices, and more
To set up an account or place an order with Stallergenes Greer, contact a Customer Care Specialist today.
For calls outside the US, dial +1.640.206.9043. To order by fax, dial 800.419.7302 or 828.759.7440.
Stallergenes Greer Allergy Products and Services Catalog

See the specifics on all our innovative tools, customizable allergen immunotherapy (AIT) extracts, and wide range of products and services—designed to help you deliver personalized care to your patients.
Choose from a wide range of extracts and supplies for practices interested in mixing in office
GREER® Extracts™

A wide selection of quality allergenic extracts, concentrated in both aqueous and glycerinated formulations
GREER® Sterile Diluents™
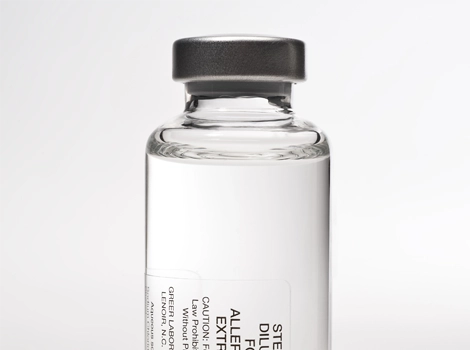
Sterile diluents, available in various sizes, formulations, and fill volumes
GREER® Sterile Empty Vials™

Industry-standard vials for extract mixing and storage in multiple sizes
GREER® Versa Vial Rack®

Portable and space-saving vial racks for organizing extracts efficiently, available in multiple styles and sizes
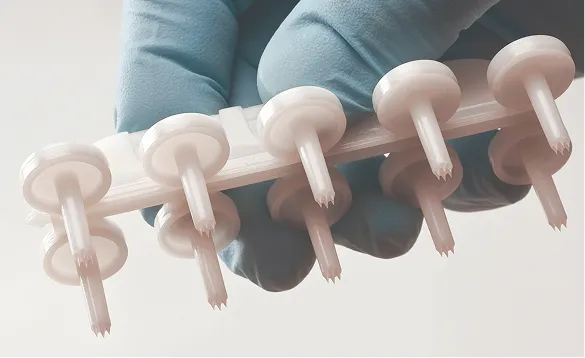
Skintestor OMNI™
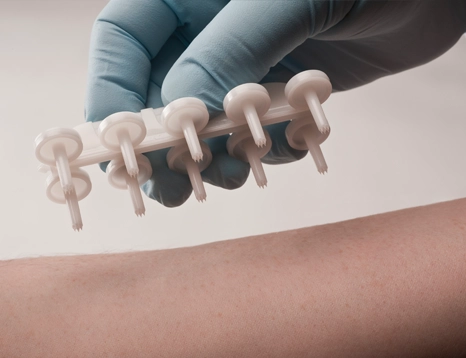
A self-loading 10-site device for testing multiple allergens at once
GREER® Pick®
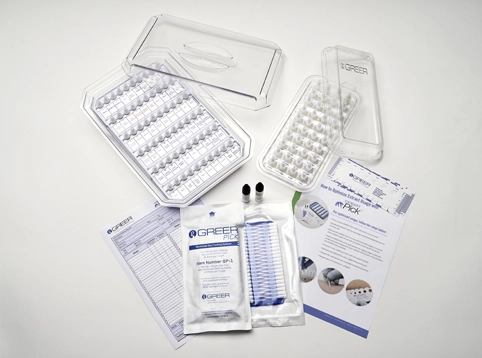
A self-loading, single-use, single-site skin testing system
Order prescription named-patient AIT formulations through GREER® Pharmacy
As you plan your practice’s future—whether evaluating AIT prescription options, relocating, remodeling, managing staff changes, or handling complex compounding needs—consider GREER® Pharmacy for its precision and reliability.
Licensed across the US
Licensed by state boards of pharmacy to dispense prescriptions throughout the US

Safety standards
Meets USP <797> standards for sterile compound formulations

Compounding you can count on
Compounding capabilities for complex, multi-allergen prescriptions
Stallergenes Greer is the only US-licensed extract manufacturer with a fully licensed compounding pharmacy.

In January, our on-time in-full (OTIF) rate was 93.2%
What does on-time in-full (OTIF) mean?
It means that customers’ orders are successfully fulfilled on the agreed-upon ship date with the quantity and specifications requested.
- On-time: all orders placed for in-stock items before 3:00pm EST are shipped the same day
- In-full: every in-stock item you ordered is included in the shipment
Why does it matter?
As a customer-centric company, it’s important to us to deliver what you need within the time frame that you need it.
Ordering information
Ordering
For quality service, specify account number, item number, product name, vial size and dilution, aqueous or glycerinated formulation, and skin prick test or multiple dose vial as shown in the catalog. Physician license must be on file. Proper identification will be required from institutions.
Minimum order
A minimum order of $25.00 is required. Please combine orders to achieve minimum.
Shipping
All extracts are shipped overnight for next-day delivery. Unless otherwise specified by customer, all other products ship via UPS 3 Day Select® or Ground. Shipments with ice packs are available upon customer request, with an additional charge.
To set up an account or place an order with Stallergenes Greer, please contact us at:
uscustomercare@stallergenesgreer.com
800.378.3906
For calls outside the US, dial +1.640.206.9043.
To order by fax, dial 800.419.7302 or 828.759.7440.