Allergy skin testing devices to help tailor treatment
From our wide range of testing extracts to flexible skin testing device options, we are here to support your patients through their allergen immunotherapy (AIT) journey. Explore our allergy skin testing devices and how to use them.
Efficient testing options for every allergy practice
GREER® Pick® and Skintestor OMNI™ are single and multisite skin testing systems designed for prick or puncture skin testing techniques.

The Skintestor OMNI™ is a self-loading device designed for quick and efficient multisite testing. Features include:
- 10 test sites per applicator for faster administration
- Large, covered wells to maintain extract integrity and prevent spills
- Options for 20 or 40 well trays, customizable to your practice needs
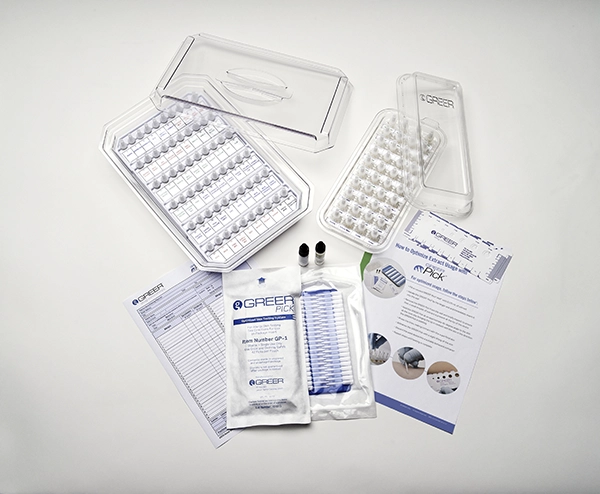

GREER® Pick®* is a self-loading, single-use, single-site skin testing system. Features include:
- Transparent trays for easy inspection of extract levels
- Options for 40 or 60 well trays for allergen panel flexibility
Refer to this helpful skin testing sheet designed for use with GREER® Pick®:
*Please see Directions for Use for GREER® Pick®.
Explore the Skintestor OMNI™
Review protocols for using GREER® Pick® and Skintestor OMNI™ devices so you can feel confident in your test results
We offer:

Review sessions
Led by an AIT team member
Schedule a Skin Testing Review Session with a Stallergenes Greer Specialist
Call to schedule a session:
800.378.3906

Instructional videos
Self-paced online review
Designed to help review proper technique for using Skintestor OMNI™ or GREER® Pick®
Sign up for online Skin Testing Review Sessions
If you’ve purchased a Skintestor OMNI™ or GREER® Pick® device, you can register below to request access to our online education videos. Everything that would be covered during a review session is covered in quick and engaging videos. A Stallergenes Greer representative may contact you if more information is needed.




